About Chemiluminescence Detection
Chemiluminescence detection is a technique that allows for detection at ultra-high sensitivities. Although there are not many examples of chemiluminescence detection being used with HPLC analysis, this article presents a basic understanding of the technique.
What Is Chemiluminescence?
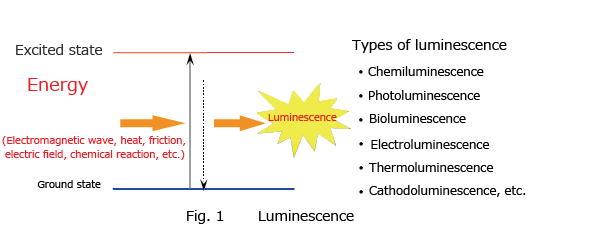
Luminescence is the phenomenon whereby matter emits light of a specific wavelength without emitting heat and returns to a ground state from an excited state after having absorbed external energy from an electromagnetic wave, heat, friction, electric field, or chemical reaction (Fig. 1). When the source of the energy absorbed is a chemical reaction, this phenomenon is called chemiluminescence. When light is the energy absorbed, the phenomenon is called photoluminescence, and this phenomenon includes fluorescence and phosphorescence. Light emission by fireflies and deep sea organisms is called bioluminescence.
Characteristics of Chemiluminescence Detection
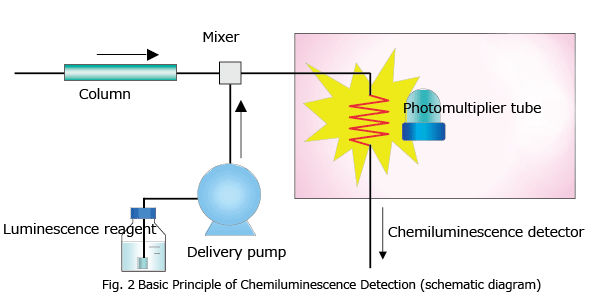
Chemiluminescence does not require a light source lamp (such as a xenon lamp) since material is excited by the energy of a chemical reaction caused by a luminescence reagent. Fig. 2 shows the basic principle of chemiluminescence detection in HPLC. As with post-column derivatization, the luminescence reagent used for this chemical reaction is continuously added to the column eluate by a luminescence reagent delivery pump, and mixed with the column eluate in the mixer. Luminescence arising from this chemical reaction is measured by a photomultiplier tube when luminescence reaches its highest intensity. A coiled tube made of easily malleable fluoroplastic is commonly used as the chemiluminescence flow cell, since it allows the photomultiplier tube to receive as much of the emitted light as possible.
Example of Chemiluminescence Reagents
There are two types of chemiluminescence reagents: direct luminescence reagents where the excited material itself emits light, and indirect luminescence reagents where energy from the chemical reaction excites another material.
- Direct luminescence type
A typical example of this type of chemiluminescence reagent is luminol, which has been used for many years to identify blood. Fig. 3 shows the chemiluminescence reaction caused by luminol. When in the presence of a metallic catalyst, luminol emits a blue light (425 nm) in response to strong alkalinity, oxidizing agents such as hydrogen peroxide, and heat. The luminol reaction can be used in HPLC to analyze peroxides (lipid peroxides, etc.) and metal-containing materials.
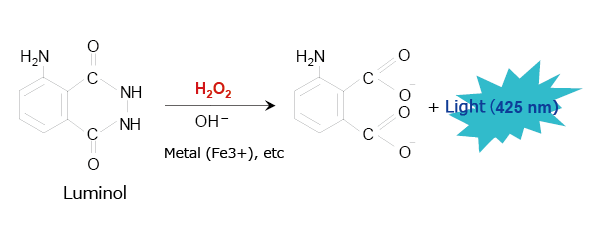
Fig. 3 Chemiluminescence Reaction Caused by Luminol
- Indirect luminescence type
A typical example of this type of chemiluminescence reagent is the combination of an oxalic acid diester and hydrogen peroxide. Fig. 4 shows the chemiluminescence reaction caused by oxalic acid bis(2,4,6-trichlorophenyl) (TCPO). In this reaction, the active intermediate (1,2-dioxetanedione) produced by the reaction between TCPO and hydrogen peroxide delivers excitation energy to the luminescent material when it decomposes into carbon dioxide.
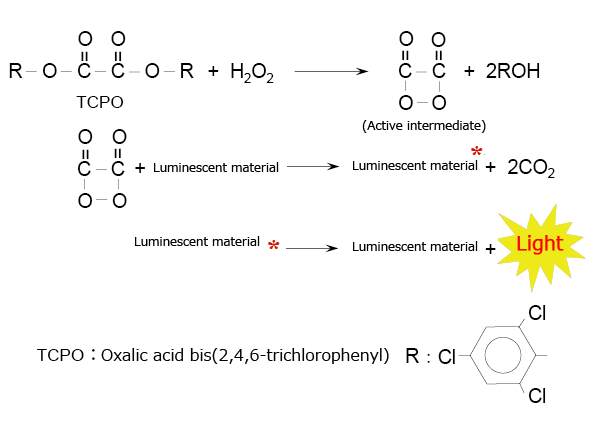
Fig. 4 Chemiluminescence Reaction Between an Oxalic Acid Diester (TCPO) and Hydrogen Peroxide
When the analyte is not a luminescent material, this method is used to convert it to a luminescent derivative. For example, when the analyte is a dansylated amino acid, the indirect chemiluminescence method allows for detection at ultra-high sensitivities in the order of femtomoles (10-15 mol) or several hundreds of attomoles (10-18 mol).


