Silica Gel Packing Material: Basic Terminology1
This article explains basic terminology related to silica gel packing material used for HPLC. It collects terms commonly used in column instruction manuals and HPLC user guides.
Silanol group
Silica gel is an amorphous silicic acid polymer represented as SiO2-nH2O. On the surface of silica gel are found silanol groups (Si-OH), of which there are three known types: isolated, vicinal (hydrogen bonded with a neighboring silanol group), and geminal (Fig. 1). Silanol groups generally exhibit weak acidity, though it can vary in strength depending on the individual state of the group.
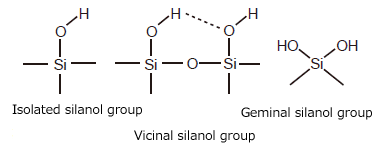
Silanol groups serve as adsorption active sites in adsorption chromatography. They are essential groups used to add stationary phase functional groups by chemical bonding, which is described below.
Chemically bonded silica gel
Chemically bonded silica gel is silica gel packing material to which a stationary phase functional group has been bonded chemically (by covalent bonding). Chemically bonded silica gel is normally made by reacting an organosilane compound that can contain various functional groups with the silanol groups on the surface of the silica gel (a reaction called silylation). ODS is an acronym for the name of a packing material used widely in reverse phase chromatography. It is silica gel to which the n-octadecyl silyl functional group has been added. An example method of preparing ODS is shown in Fig. 2.
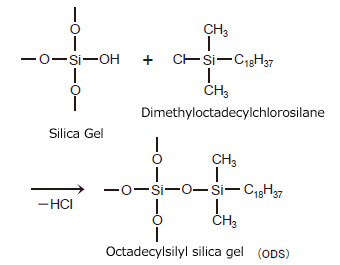
Fig. 2 Example Method of Preparing ODS
Monomeric stationary phase
Polymeric stationary phase
When preparing chemically bonded silica gel, if the organosilane molecule contains a single reactive functional group as shown in Fig. 1 (a Cl group in this case), the organosilane reacts with a single silanol group. This creates what is called a monomeric stationary phase. When the organosilane molecule contains two or three reactive functional groups, it reacts with other additional silanol groups or organosilane molecules. This creates a polymeric stationary phase. Chemically-bonded phases can vary depending on the synthesis conditions. An example ODS prepared from an organosilane that contains three functional groups (octadecyltrichlorosilane) is shown in Fig. 3. In addition to this example, there are other polymeric stationary phases that contain even more silyl groups, and that have functional groups that bond to silica gel at three sites. Chemically bonded silica gel that contains polymeric bonding mixed with monomeric type bonding to a single silanol group also exists, as shown in Fig. 2. (When this occurs, the remaining two Cl groups become OH groups.)
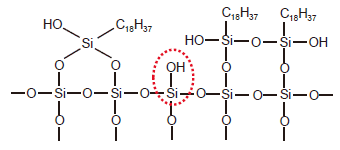
Fig. 3 ODS Prepared from an Organosilane That Contains Three Functional Groups
Generally, monomeric stationary phases are easy to synthesize and their synthesis is highly reproducible, while their drawbacks include poor durability and poor retention capacity. Meanwhile, polymeric stationary phases are very difficult to synthesize, but many different shapes and stationary phase functional groups can be introduced depending on the synthesis conditions. Furthermore, polymeric stationary phases have good retention capacity and are highly durable.
However, neither type is necessarily better than the other, since which type is most appropriate depends on the analysis being performed. Characteristic differences are also present between manufacturers, and packaging material selection requires a certain amount of trial and error and use of technical materials and data provided by the manufacturer.
Endcapping
The preparation of chemically bonded silica gel always leaves behind unreacted silanol groups due to steric hindrance and other causes (called residual silanol groups, one circled in red in Fig. 3). While residual silanol groups can have a positive effect on separation, in most cases they cause peak tailing of basic compounds. Consequently, after the introduction of stationary phase functional groups, residual silanol groups are then silylated. This process of silylation is called endcapping. Removing every residual silanol group is difficult even with endcapping, and each manufacturer has their own method for dealing with this issue.
Reference:
1) JIS K 0214:2013 Technical terms for analytical chemistry (Chromatography part)


