Important Points Related to KBr Pellet Method
分子光譜
- Samples That Cannot be Analyzed by KBr Pellet Method (Hydrochlorides) -
Many people know that since the Fourteenth Edition of the Japanese Pharmacopoeia, the general test method changed from the potassium bromide (KBr) pellet method to a choice between the KBr pellet method or the potassium chloride (KCl) pellet method. But it is not well known why the prescribed test method was changed or which test method should be applied to what sample. This Application News compares the KBr pellet method and the KCl pellet method on hydrochlorides, which are typical samples that should not be analyzed using the KBr pellet method.
■ Pelletizer (miniature manual press)
Fig. 1 shows the MHP-1 pelletizer used to prepare the test samples. The accessories supplied make it extremely easy to form 4-mm dia. pellets (previously 3-mm dia.). Unlike conventional pelletizers, it requires no vacuum pump. It is a manual press that can rapidly form the pellets.
In accordance with the Japanese Pharmacopoeia, the wavenumber range was set to 4000 to 400cm-1 for the measurements. Table 1 shows the measurement conditions in detail.
Table 1 Measurement Conditions
- Resolution : 4 cm-1
- Accumulation : 45
- Detector : DLATGS

Fig 1 Appearance of MHP-1 Pelletizer
■ Measurement of L-Cysteine Hydrochloride
Fig. 2 shows the spectrum measured by the KCl pellet method (blue) and the spectrum measured by the KBr pellet method (red). Fig. 3 shows a magnified image of Fig. 2 in the range from 900 to 700 cm-1.
Fig. 3 shows that the KBr pellet method spectrum exhibits a peak at 800 cm-1 where no peak should exist. In addition, the peak position near 775 cm-1 is shifted somewhat toward the low-wavenumber end in the KBr pellet method spectrum. The difference between the two spectra results from ion exchange between chloride ions and bromide ions when a hydrochloride sample is diluted by KBr. While not shown here, the single-reflection ATR attachment can obtain a spectrum equivalent to the KCl pellet method, without pretreatment.
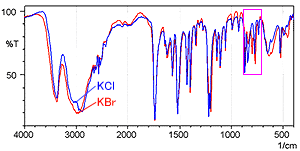
Fig. 2 Spectra of L-Cysteine Hydrochloride
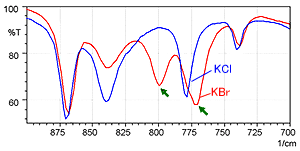
Fig. 3 Magnified View of Fig. 2
Fig. 4 shows a time series of spectra measured for L-cysteine hydrochloride using the KBr pellet method (with the same wavenumber range as Fig. 3). The blue line is the spectrum immediately after forming the pellet, the red line was measured after 2 minutes, and the green line was measured after 10 minutes.
Fig. 4 shows that the peak intensity near 800 cm-1 gradually increases, while the peak near 840 cm-1 decreases. In addition, it can be seen that the peak near 775 cm-1 gradually shifts toward the low-wavenumber end. This also indicates a gradual ion exchange when L-cysteine hydrochloride is analyzed using the KBr pellet method.
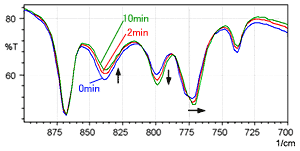
Fig. 4 Spectrum of L-Cysteine Hydrochloride (KBr Pellet Method)
■ Measurement of Diphenhydramine Hydrochloride
The Japanese Pharmacopoeia prescribes the KCl pellet method for the analysis of diphenhydramine hydrochloride, instead of the KBr pellet method. Here, we compare the differences between analysis by these two methods.
Fig. 5 shows the spectra of diphenhydramine hydrochloride measured using the KBr pellet method and the KCl pellet method. The red line is the KBr pellet method spectrum and the blue line is the KCl pellet method spectrum.
The shapes of the spectra are clearly different near 3000 cm-1. Other differences are also apparent in the low-wavenumber region. Fig. 6 shows an magnified image of the 1500 to 400 cm-1 wavelength range where differences are apparent.
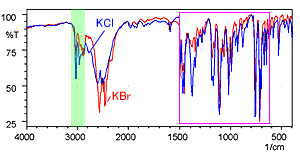
Fig. 5 Spectra of Diphenhydramine Hydrochloride
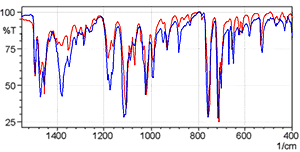
Fig. 6 Magnified View of Fig. 5
Observing the data for the single-reflection ATR measurement of diphenhydramine hydrochloride in Application News A323 (pdf, 40 kB) clearly indicates that the KBr pellet method spectrum is distorted due to ion exchange. However, the KCl pellet method obtains a spectrum similar to the single-reflection ATR method. Should ion exchange occur with the sample during the KCl pellet measurements, as the ions exchanged are the same chloride ions, it results in no change to the sample structure.
As shown above, as ion exchange occurs in some hydrochloride samples when analyzed using the KBr pellet method, the results of analysis by the KBr pellet method must be confirmed by some other analysis method.


