Chapter 1: What is Thermal Analysis?
Thermal analysis is a general term defining a technique used to analyze the time and temperature at which physical changes occur when a substance is heated or cooled. Each technique is defined according to the types of physical changes being analyzed. When evaluating material characteristics, it is necessary to use different techniques or a combination of multiple techniques depending on the purpose.
Definition of Thermal Analysis (ICTAC)
The study of the relationship between a sample property and its temperature as the sample is heated or cooled in a controlled manner.
References: ICTAC (IUPAC Recommendations 2014)
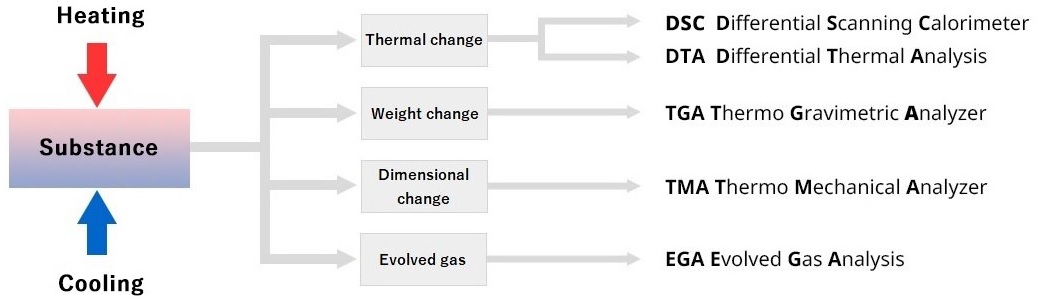
Measuring methods for thermal analysis of physical properties
Several measuring methods are available. The method used varies according to the physical properties being analyzed. The most commonly used ones are as follows:
| Physical Properties | Types of Obtainable Information | Measuring Methods | Units |
|---|---|---|---|
| Calorie | Transition Temperature, Heat Capacity Transferred, Specific Heat Capacity, Reaction Temperature, Reaction Calorie, Examination of Thermal History etc. | Differential Scanning Calorimetry DSC |
mW(=J/s) |
| Temperature | Transition Temperature, Reaction Temperature etc. | Differential Thermal Analysis DTA |
μV |
| Mass | Dehydration, Oxidation, Pyrolysis, Evaporation, Sublimation etc. | Thermo Gravimetric Analysis TGA |
mg |
| Dimension | Thermal Expansion, Thermal Shrinkage, Glass Transition Temperature, Softening Temperature etc. | Thermo Mechanical Analysis TMA |
μm |
Each technique is used to measure different phenomena and physical properties.
| Name of Techniques |
Applicable Shimadzu products |
Measuring Objects for Phenomena and Physical Properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Melting | Glass Transition |
Crystallization | Reaction (Curing and Polymerization) |
Sublimation, Evaporation, Dehydration |
Pyrolysis | Thermal Expansion, Thermal Shrinkage |
Examination of Thermal History | Specific Heat Capacity | ||
| DSC | DSC-60 Plus | √ | √ | √ | √ | √*1 | √*1 | - | √ | √ |
| DTA | DTG-60 | √ | √ | √ | √ | √ | √ | - | √ | √ |
| TG (TGA) |
TGA-50 | - | - | - | √*2 | √ | √ | - | - | - |
| DTG-60 | ||||||||||
| TMA | TMA-60 | √*3 | √ | - | √ | - | - | √ | √ | - |
*1 Since the thermocouple of DSC is weak to corrosive gas, decomposition reaction is not usually measured.
*2 The measuring object is the one which varies the weight with the reaction.
*3 Detects as softening.
Note:
- DTG is a simultaneous measuring device consisting of TG and DTA.
- DSC can measure transitions (melting, glass transition, crystallization) and reactions, such as hardening, as well as specific heat capacity, and also examine thermal history.
- In order to avoid contamination and corrosion of the device, DSC is not usually used for measurements in which gases are generated by decomposition reactions.
- TG can measure phenomena with weight changes, such as sublimation, evaporation, dehydration, and pyrolysis.
- By conducting a simultaneous measurement using TG and DTA, the thermal change of the sample can be estimated.
- TMA can measure phenomena with dimensional changes such as glass transition, thermal expansion, softening, and examination of thermal history. Melting is usually measured as softening because a solid sample softens (flows).
The optimum measurement mode (Expansion, Tension, Needle Insertion, etc.) needs to be selected according to the sample shape, type and purpose of measurement.


