What Is Light
The question of whether light is wave or particle, throughout history, been the subject of much debate. It is well known that various theories developed from Newton's assertion that light is particle and Huygen's assertion that light is wave. In modern physics, it is now known that; while light has the wave nature, it also has particle-like properties. In this article, we will describe the dual nature of light and the relationship between matter and light absorbance.
1. The Dual Nature of Light
(1) Wave-Like Properties of Light
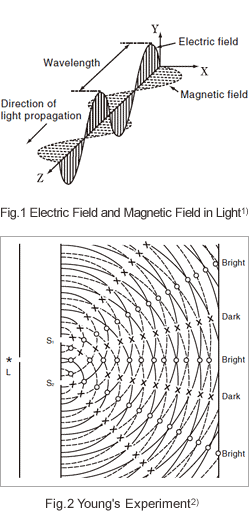
Although light is generally said to be a wave, unlike the waves that occur at the surface of a body of water, it does not require a medium. As shown in Fig.1, light consists of an electric field and a magnetic field that intersect each other at a right angle as they move through a vacuum. The distance between successive peaks of either the electric field or the magnetic field is the wavelength.
When handling light, one encounters phenomena that are particular to waves, such as interference and diffraction. The experiment in which Young discovered the interference of light and concluded that light was a waveform is well known. As shown in Fig.2, the monochromatic light emitted from a light source, L, passes through a single slit, and then passes through two more slits, S1 and S2. As a result, interference fringes are observed on the screen at the back as a pattern of alternating strips of brightness and darkness. This can be explained by thinking of S1 and S2 as light sources in phase with each other. Waves travel from these light sources to the screen at the back. At points where the waves are in phase, they reinforce each other, whereas at points where the waves are out of phase, they cancel each other out. If one thinks of the surface of this paper as the surface of a body of water, and the slits as partitions with holes in them, then waves moving from left to right would behave in the same way. In this sense, Young's experiment demonstrates the wave-like nature of light in an intuitive way. Incidentally, the diffraction grating used in a UV-VIS spectrophotometer creates monochromatic light using the wave nature that light diffracts and causes interference.
Light consists of certain types of electromagnetic waves.
Electromagnetic waves are referred to by different names in accordance with their wavelength, as shown in Fig.3. "Light" usually refers to electromagnetic waves in the range spanning infrared radiation and ultraviolet radiation, but in some cases it refers only to visible light. Light with wavelengths in a range of approximately 400 to 800 nm is referred to as "visible light", and is the light that we humans can see with the naked eye.
For example, light with a wavelength of 470 nm is blue, light with a wavelength of 540 nm is green, and light with a wavelength of 650 nm is red. Visible light could be described as the kind of light that we humans are familiar with because of our ability to actually see it.
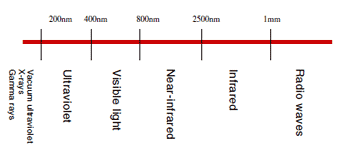
Fig.3 Classification of Light According to Wavelength
(2) Particle-Like Properties of Light
Next, let us look at the particle-like behavior of light. Among the developments that helped identify this behavior was a series of experiments on the photoelectric effect that were conducted in the late 19th century and the early 20th century.
The results of these experiments could not be explained by considering light as a wave, but they could be explained by considering it as a particle. When emphasizing the particle-like aspects of light, the term "photon" is used.
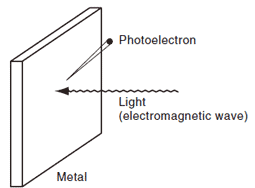
Fig.4 Concept of Photoelectric Effect3)
Fig.4 illustrates the basic concept of the photoelectric effect, a phenomenon in which electrons are emitted from a metal surface when light strikes it. The emitted electrons are called "photoelectrons". Even though no electrons are emitted when intense light strikes the surface, electrons are emitted when light of a shorter wavelength strikes the surface. If the wavelength of the light striking the surface is decreased, the number of electrons emitted does not change, but the energy of the electrons increases. If the light striking the surface is intensified, the number of emitted electrons increases, but the energy of the electrons stays the same. These phenomena cannot be explained by considering light as a wave, but they can be explained by considering it as a particle, with electrons being knocked out by these particles when they strike the metal. In combination with results of experiments on the Compton effect and other experiments, the particle-like properties were recognized. Incidentally, the photomultiplier tube that is used as the detector in UV-VIS spectrophotometers detects light using the photoelectric effect.
We have looked at the dual nature of light, with its mixture of wave-like and particle-like properties. The fact that light has these two opposing characteristics may seem strange, but this is the way light is modeled in modern physics.
2. Absorption of Light by Matter
A wide variety of information about a substance can be obtained by irradiating it with light. With a UV-VIS spectrophotometer, irradiating a substance with ultraviolet and visible light makes it possible to obtain information about the electrons in that substance, and it is even possible to measure its quantity.
Let us consider the absorption of light by matter. This is closely related to quantum mechanics. The theory of quantum mechanics was developed in the early 20th century, and forms part of the basis of modern physics. Quantum mechanics can be easily understood by comparing it to Newtonian mechanics.
Broadly speaking, Newtonian mechanics is a theory that relates to the motion of large particles, whereas quantum
mechanics is a theory that relates to the motion of small particles (e.g., atoms and molecules). Newtonian mechanics handles the motion of particles as a continuous entity, whereas quantum mechanics asserts that small particles exist in discrete states of motion (energies). At the time when Newtonian mechanics was the dominant theory, the concept of quantum mechanics was difficult for people to accept. Over time, however, its validity was demonstrated.
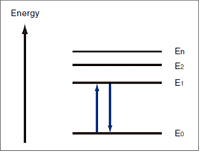
Fig.5 Energy Levels
Solving the equations of quantum mechanics that relate to the electrons in an atom gives a model, like that shown in Fig.5, in which the electrons have discrete energy states. E0 is called the "ground state" and E1, E2, etc., are called "excited states".
In order for an electron to switch from E0 to E1, light with an energy of (E1 - E0) must strike the electron. This is the "absorption" of light. Electrons have particular energy levels,and rays of ultraviolet and visible light have the energy to change the energy states of the electrons.
Because the higher energy state, E1, is unstable, the electron soon returns to the ground state, E0. The energy discharged when the electron returns from E0 to E1(E1 - E0) is converted to heat. If, for some reason, it is not converted to heat, the energy is discharged as light. The phenomenon of light emission is well known as fluorescence or phosphorescence.
In relation to quantitative measurement performed with spectroscopy, the consequence of this phenomenon is that there is a large amount of absorption if a large number of target molecules exist in a solution, and only a small amount of absorption if there is only a small number of target molecules.
Obtaining the quantity, and thereby the concentration, of a substance from the degree of absorption is the fundamental principle behind quantitative measurement.
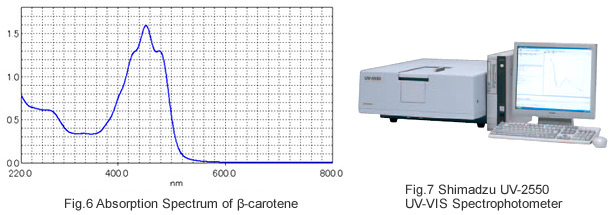
The graph obtained by using the horizontal axis to represent the wavelength and the vertical axis to represent the degree of absorption is called an "absorption spectrum". The degree of absorption is expressed in terms of the unit "absorbance"(Abs). Fig.6 shows an absorption spectrum of β-carotene solution obtained with the Shimadzu UV-2550 UV-VIS spectrophotometer (Fig.7). β-carotene is the principle substance in carrots that gives them their color. As shown in Fig. 6, mainly blue and purple light of wavelengths in the range of 400 to 500 nm is absorbed. Because the visible light that reaches the eye of the observer consists of a mixture of the green and red components that are left over, carrots, which contain a large amount of β-carotene, appear to have a yellowred
coloring.
3. Summary
Here, we have looked at the properties of light and the way that it is absorbed, two fundamental aspects of the operation of a UV-VIS spectrophotometer. In the field of spectroscopy, in addition to UV-VIS spectrophotometers, there are various other types of spectroscopic measurement devices, such as infrared spectrophotometers, atomic absorption photometers, Raman spectrophotometers, and fluorescence spectrophotometers, all of which perform distinctive types of analysis. Using these devices selectively makes it possible to obtain various types of information about samples from different perspectives. In the next volume, we will describe the structure of a UV-VIS spectrophotometer.
- 1) Kanji Kihone: Measuring Light, Chapter 10
(Edited by the Illuminating Engineering Institute of Japan,Published by Nippon Riko Shuppankai, 1993), p. 172 - 2) Akira Harajima: Elementary Quantum Mechanics
(Shokabo, 1987), p. 3 - 3) Ryuzo Abe: Introduction to Quantum Mechanics
(Iwanami Shoten, Publishers, 1987), p. 31
The booklet addresses a range of UV related topics and useful analysis information and know-how using Shimadzu UV spectrophotometers.


