The Horror of Sample Adsorption to Containers (Part 2)
Naoki Asakawa,
Technical Advisor, Analytical & Measuring Instruments Division,
Shimadzu Corporation
Part 1 gave an outline of mechanisms of sample adsorption to containers and how to reduce this adsorption. Part 2 describes the characteristics of LabTotal Vial (glass) and TORAST™-H Bio Vial (polypropylene) low-adsorption vials developed based on mechanisms of container adsorption, and the adsorption-reducing effect of these vials on basic compounds and peptides.
(1) LabTotal Vial
LabTotal Vial is a vial that reduces the adsorption of basic compounds to its glass vial surface. Fig. 1 shows chromatograms obtained from investigating the effects of dispensing a neutral compound and basic compound mixture into LabTotal Vial and commercially available glass vials (A, B).
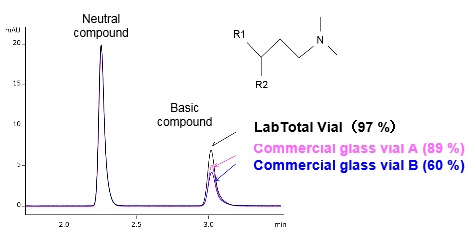
Fig. 1 Effect of LabTotal Vial
The percentage peak area for each vial type compared to a polypropylene (PP) vial (100 %) is shown in brackets. The results confirm the peak area of the basic compound is reduced when commercially available glass vials are used. As described in Part 1, basic compounds tend to adsorb to glass surfaces by interaction with silanol groups. This adsorption can be reduced by adjusting the sample solvent, but by reducing adsorption at the vial surface, LabTotal Vial precludes the need to consider adjustments within the sample solvent.
Table 1 shows a comparison between LabTotal Vial and commercially available glass vials for four different basic compounds. Similar to Fig. 1, the results confirm the effectiveness of LabTotal Vial with multiple basic compounds.
Part 1 described methods of reducing adsorption based on the physical characteristics of target components, but such methods are difficult to apply when analyzing unknown compounds and compounds that contain a mixture of physical characteristics.
Table 1 Comparison of Vials with Four Different Basic Compounds
| Compound* | Amitriptyline | Atenolol | Imipramine | Propranolol |
|---|---|---|---|---|
| LabTotal Vial | 51376 (100 %) |
8638 (100 %) |
64990 (100 %) |
32249 (100 %) |
| Vial A | 45376 (88 %) |
7620 (88 %) |
55531 (85 %) |
31496 (97 %) |
| Vial B | 21788 (42 %) |
6137 (71 %) |
24131 (37 %) |
27327 (84 %) |
(Top: peak area, bottom: percentage peak area for each vial compared to LabTotal Vial [100 %])
Fig. 2 compares the results obtained from using LabTotal Vial, a PP vial, and a commercially available glass vial B with amiodarone, a compound that has both hydrophobic and basic properties.
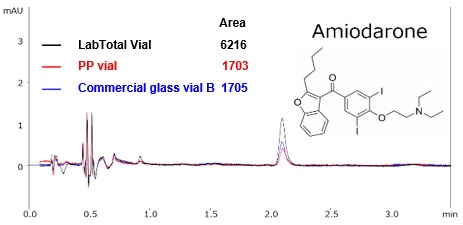
Fig. 2 Comparison of Vials with Amiodarone*
For the PP vial, peak area is speculated to have decreased due to hydrophobic adsorption, and with the glass vials, peak area is presumed to have decreased due to both hydrophobic adsorption and ionic adsorption. Assuming a constant quantity of target component is adsorbed to the vial (vials are manufactured homogeneously with no variation in vials), the issue of adsorption becomes more noticeable at lower concentrations, which then reduces calibration curve linearity. LabTotal Vial can be positioned for use as a vial of first choice since it reduces adsorption and precludes the need to choose between different vial types based on physical characteristics or concentration of the target component.
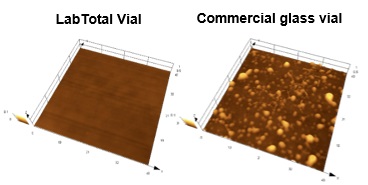
Fig. 3 Results of Surface Observations
Optimization of LabTotal Vial molding conditions is speculated to standardize conditions at the glass surface and make adsorption more difficult. In view of this, results of vial surface observations that were performed to determine the reason for reduced adsorption exhibited with LabTotal Vial are shown in Fig. 3.
Comparing the surface conditions of the vials shows the surface of LabTotal Vial is smoother than the commercially available vial. Adsorption to the commercially available glass vial is conjectured to be more likely to occur because a greater surface area is contactable by the sample. In the future, I aim to perform further chemical analysis of the vial surface and further determine the mechanism by which adsorption is reduced.
(2) TORAST-H Bio Vial
TORAST-H Bio Vial is a low-adsorption vial with hydrophilic groups chemically bound to its surface. The main mechanisms of peptide adsorption to vials are regarded as hydrophobic adsorption and ionic adsorption. Adsorption to glass vials is known to be mainly by ionic adsorption to silanol groups at the glass surface, and adsorption to polymer vials such as PP vials is known to be mainly by hydrophobic adsorption to the polymer surface.
Fig. 4 shows a comparison between different types of vials using a tryptic digest of myoglobin as the sample. The results confirm a large quantity of highly polar peptides that were detected at a retention time of around seven to eight minutes adsorbed to the glass vial, and the marked adsorption of highly hydrophobic peptides detected at a retention time of around 12 to 17 minutes to the PP vial. The results also confirm adsorption of these peptides was reduced in TORAST-H Bio Vial.
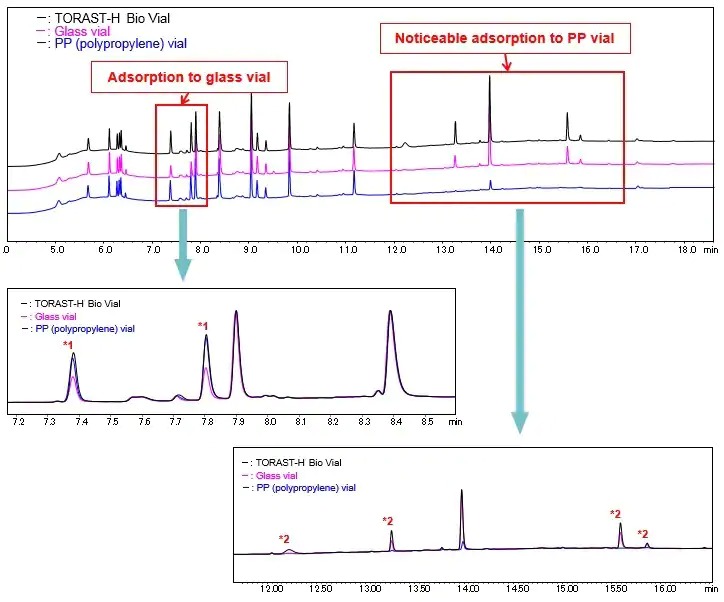
Fig. 4 Comparison of Each Vial Using Tryptic Digest of Myoglobin
For peaks labeled *1, the results confirm using TORAST-H Bio Vial improved recovery by approximately 40 % over the glass vial (peak area comparison). For peaks labeled *2, the peaks were almost undetectable using the PP vial while the peaks were detectable using TORAST-H Bio Vial.
In light of the above, it goes without saying that in the pursuit of highly sensitive quantitative analysis by methods such as LC/MS, the phenomenon of container adsorption during sample preparation is a serious issue in ensuring reliability of analytical methods. I hope this article will be of assistance to many analysts. Shimadzu Group continues to investigate reduction of adsorption during the sample preparation through sample measurement processes for the purpose of ensuring analytical method reliability, and I look forward to describing the results of our investigations in the near future.
* The comparison using amiodarone (Fig. 2) is based on data obtained in joint study with the Division of Pharmacy, National Cerebral and Cardiovascular Center.
>>The Horror of Sample Adsorption to Containers Part 1
>>The Horror of Sample Adsorption to Containers Part 3
For more details, visit



