Switching the Mobile Phase from Acetonitrile to Methanol
If possible, attempts to switch the mobile phase from acetonitrile to methanol should be considered.
The differences in elution selectivity and other reasons might cause changes in separation and analysis conditions. When switching to methanol, pay attention to the following points.
1) Absorption
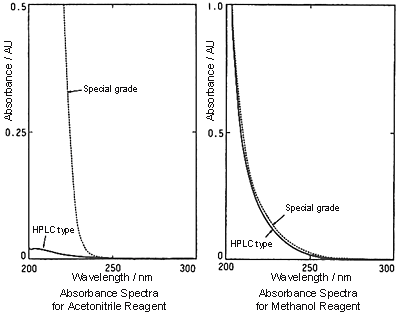
Because the absorption of methanol in the UV short-wavelength range is larger than that of acetonitrile, the baseline might become more difficult to stabilize or noise might increase depending on the detection wavelength. During gradient analysis, ghost peaks are larger than when acetonitrile is used.
2) Elution strength
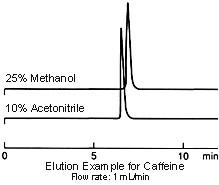
Elution strength differs with acetonitrile and methanol and, if mixed with water-based solvents, acetonitrile exhibits the greater elution strength. When conditions using acetonitrile are substituted for use with methanol, try increasing the ratio of organic solvent.
On the other hand, when the ratio of organic solvent is 100% or very close to this, the nature (polarity) of the solvent itself comes to the fore and the elution strength of methanol is greater.
3) Separation (elution) selectivity
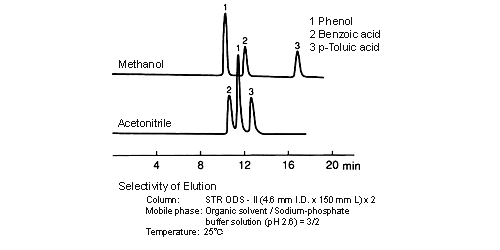
Separation selectivity differs with acetonitrile and methanol, and the degree of separation and elution order sometimes changes. A cause of this is considered to lie in the difference of the chemical nature (methanol: protic, acetonitrile: aprotic) of organic solvent molecules. When the method is changed, care must be paid to whether separation will be affected; however, in some cases, components that cannot be separated by water/acetonitrile solvents can be separated by water/methanol solvents.
4) Pressure
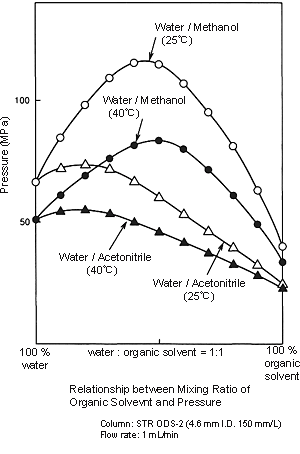
Because the viscosity of methanol is higher than that of acetonitrile, the pressure applied on the column by water/methanol solvents is higher than that of water/acetonitrile solvents. When the mobile phase is changed from a water/acetonitrile solvent to a water/methanol solvent, initially allow the solvent to flow at a lower flow rate, and then determine the analysis flow rate while checking the delivery pressure. (Before you use methanol, check the Column's Instruction Manual to ensure it can be used.) Because this will mean increasing the organic solvent ratio more than that of water/acetonitrile solvents, special attention must be paid when the methanol ratio is between 40 and 60%.


